Under the auspices of the Nancy and Stephen Grand Technion Energy program, Technion researchers have developed an innovative, clean, inexpensive, and safe technology for producing hydrogen. The technology significantly improves the efficiency of hydrogen production, from approximately 75% using current methods to an unprecedented 98.7% energy efficiency.
The Need for Hydrogen
Approximately 65 million tons of hydrogen are produced worldwide each year — the equivalent of roughly 2,600 terawatts per hour (TWh). For context, one terawatt hour could simultaneously power about 10 billion 100-watt light bulbs for an hour.
However, approximately half of the hydrogen produced today is used to make ammonia for fertilizers and other substances. The remainder is used by refineries, in methanol production, and in other ways. But in the future, we expect to see demand grow for other applications: As fuel to power fuel cell electric vehicles (FCEV), for storing energy from renewable energy sources, to heat homes and industries, and more.
Yet about 99% of the hydrogen produced today originates in fossil fuels, mainly by extracting hydrogen from natural gas. This process releases approximately 10 tons of CO2 for every ton of hydrogen extracted — making it responsible for nearly 2% of all human-made CO2 emissions into the atmosphere.
The Goal: A Cheaper, Cleaner Way to Produce Hydrogen
Technion Professor Avner Rothschild, of the Department of Materials Science and Engineering, and Professor Gideon Grader, dean of the Faculty of Chemical Engineering, together with Dr. Hen Dotan and doctoral student Avigail Landman, knew that with the demand for hydrogen increasing in the coming years, a cleaner and more environmentally friendly way to produce hydrogen had to be found.
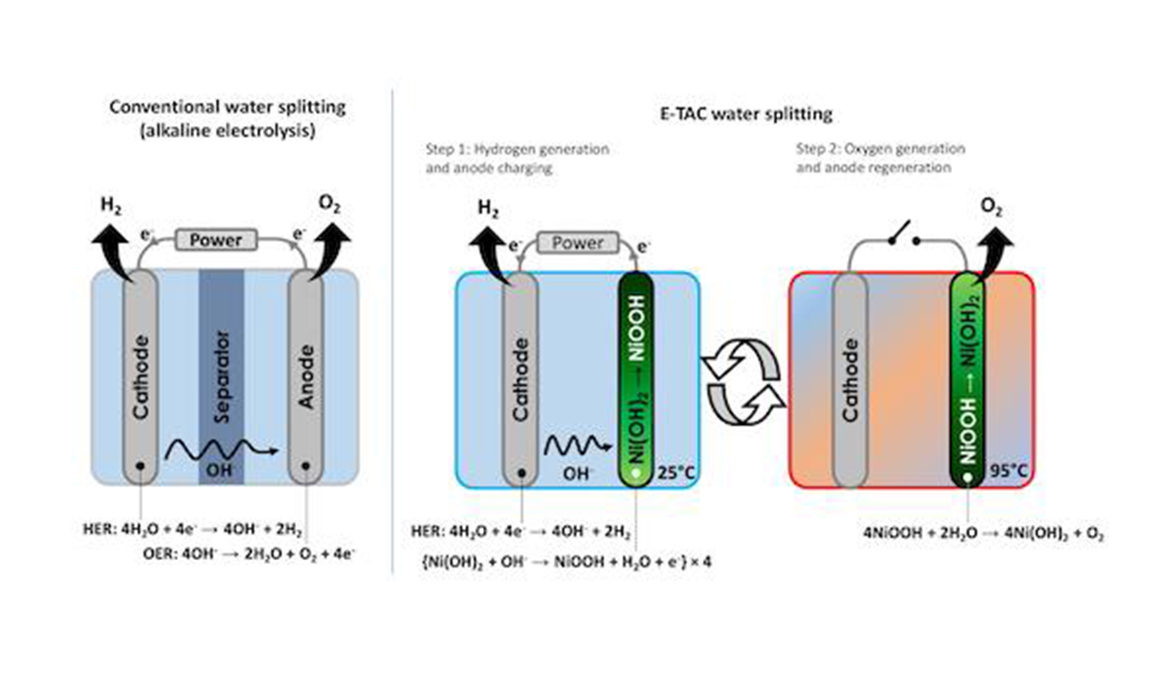
A way to produce hydrogen without CO2 emissions already existed: water electrolysis. But water electrolysis loses a lot of energy in the process, making it very inefficient. It also requires large, expensive compressors to increase the pressure of hydrogen cells and make them suitable for use in things like FCEVs.
Electrochemical–Thermally Activated Chemical Water Splitting
The researchers’ new innovative technology, called Electrochemical–Thermally Activated Chemical (E-TAC) water splitting, could be the fuel of the future. E-TAC is based on a cyclical process that, unlike water electrolysis, decouples the hydrogen and oxygen evolution reactions.
By separating hydrogen production and oxygen production so that they occur at different times, the Technion researchers found they can simplify the production process and make it both more efficient and less expensive. This process also allows hydrogen to be produced under much higher pressure, eliminating some of the high costs of compressing the hydrogen later.
And by producing oxygen via a spontaneous chemical reaction — rather than by using an electrical current — they can dramatically increase the energy efficiency of the process to an unprecedented 98.7% efficiency.
A Hydrogen Revolution
Researchers expect that E-TAC technology will cut the equipment costs of producing hydrogen in half, in addition to lowering operating costs. This could lead to a revolution in hydrogen production based on clean and renewable energy such as solar energy or wind power.