Breakthrough in Reconstructive Bone Surgery

Going back as far as World War I, surgeons have been using the so-called “Robbing Peter to Pay Paul” principle to do reconstructive surgery. That means transplanting a patient’s healthy body tissue from one part of the body to the damaged site. In rebuilding the lower jaw, for example, surgeons might use part of a patient’s fibula bone and surrounding soft tissue for the implantation.
But this approach carries significant disadvantages, namely considerable pain and potential complications associated with the surgery at the donor site. Now Technion researchers have made a major breakthrough in developing an alternative to traditional tissue replacement. Their method would help build new tissue in the lab from scratch for implantation, a process known as de novo generation. No longer would surgeons have to relocate the tissue from another part of the patient’s body.
The discovery is rooted in Professor Shulamit Levenberg’s Stem Cell and Tissue Engineering Laboratory, which focuses on creating lab-grown tissues and the complex blood vessel networks needed to nourish them. Last year, Prof. Levenberg, dean of the Technion Faculty of Biomedical Engineering, and her team demonstrated that by using these methods they could promote regeneration of spinal cord injuries in rats.
Prof. Shulamit Levenberg

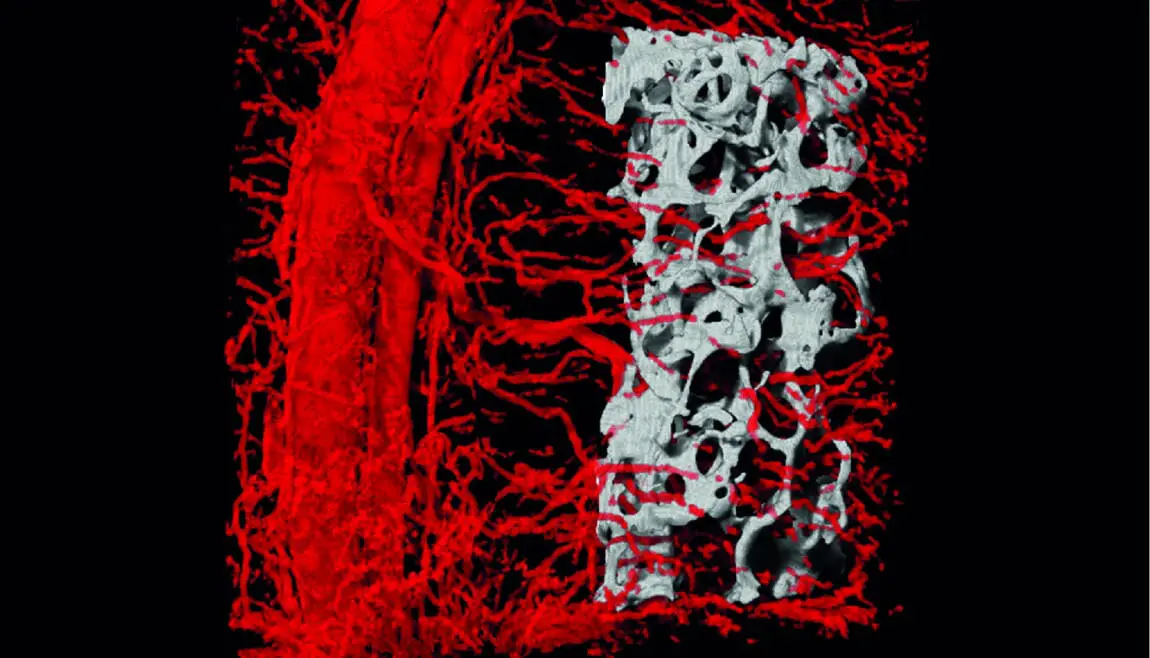
Building on that work, Dr. Idan Redenski conducted a recent study to advance these methods in bone implantation. His team put together their own technology with biological bone implants developed at Columbia University to create a de novo tissue flap containing live bone supported by vascularized soft tissue.
Dr. Idan Redenski
They then used the new methodology to repair a bone defect in rats. The rat recovered completely, and its cells grew around and replenished the implant. The researchers’ study, published in Advanced Functional Materials, took the concept of implantable bone tissue to a whole different level — demonstrating the success of reconstructive surgery without damage to other parts of the patient’s body.
Returning to the idea of a jaw implant, the researchers hope that in the near future a patient will be able to receive a lab-grown bone perfectly matching the shape of his face, surrounded by lab-grown soft tissues cultivated on 3-dimensional biomaterials.
A 3-dimensional CT scan depicting blood vessels penetrating into the embedded bone, grown within the engineered flap.